Open Access

Purushotham Endla

Endla Akhil Balaji
- Professor Department of Physics, School of Sciences Telangana India
- Student Computer Science and Engineering Telangana India
Abstract
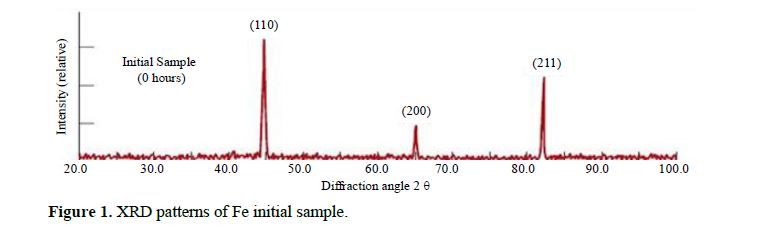
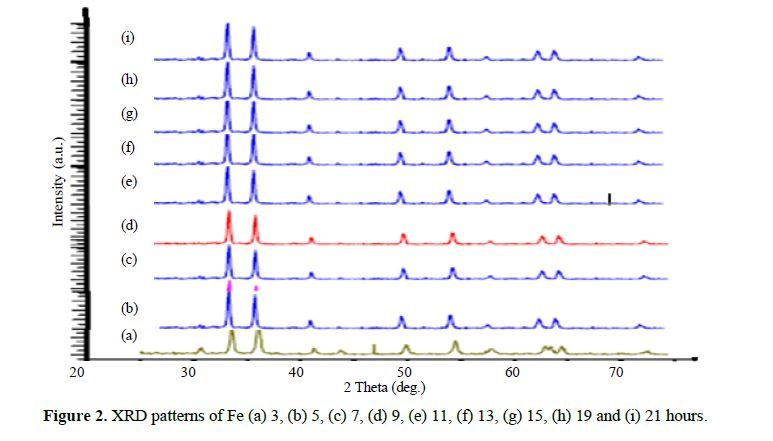
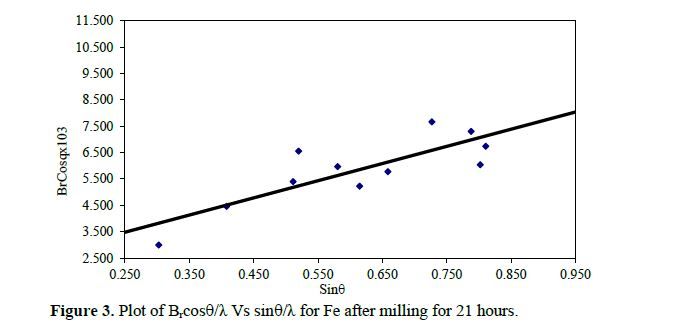
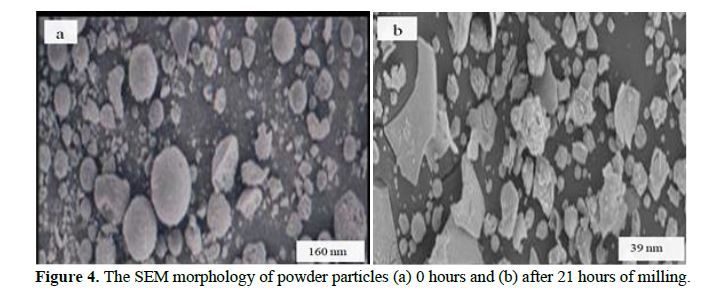
This study systematically investigates the influence of lattice strain and particle size in iron powder produced through milling, employing X-ray powder diffraction. The determination of lattice strain (ε) and the Debye-Waller factor (B) is accomplished by analyzing Bragg reflection half-widths and integrated intensities. Significantly, the Debye-Waller factor exhibited a notable increase with lattice strain. By establishing a correlation between stress and the influential Debye-Waller factor, we derived a precise relationship tailored to Fe. Additionally, we delved into the variation of the vacancy formation energy as a function of lattice strain, thereby unveiling crucial insights into the mechanistic aspects governing Fe powder behavior during mechanical milling. This investigation enhances our understanding of Fe’s structural properties and offers novel perspectives for optimizing milling processes in diverse industrial applications.
Keywords: X-ray diffraction, lattice strain, particle size, Debye-Waller factor, vacancy formation energy
[This article belongs to Special Issue under section in Journal of Polymer and Composites(jopc)]

Browse Figures
References
- Singh, et al. Optimization of synthesis parameters of silver nanoparticles and its antimicrobial activity, Mater. Sci. Energy Technol., 3 (2020), pp. 232–236
- Vallabani NVS, Singh S (2018) Recent advances and future prospects of iron oxide nanoparticles in biomedicine and diagnostics. 3 Biotech 8:2–23. https://doi.org/10.1007/s13205-018-1286-z
- Wei QY, He KM, Chen JL, Xu YM, Lau A (2019) Phytofabrication of nanoparticles as novel drugs for anticancer applications. Molecules 24(23):1–44. https://doi.org/10.3390/molecules24234246
- Vasantharaj, S. et al. Synthesis of ecofriendly copper oxide nanoparticles for fabrication over textile fabrics: Characterization of antibacterial activity and dye degradation potential. Photochem. Photobiol. B Biol. 191, 143–149 (2019).
- Sravanthi, Green synthesis, characterization and catalytic activity of 4-nitrophenol reduction and formation of benzimidazoles using bentonite supported zero valent iron nanoparticles, Mater. Sci. Energy Technol., 2 (2) (2019), pp. 298-307
- Saif, et al., Green synthesis of iron nanoparticles and their environmental applications and implications, Nanomaterials, 6 (11) (2016), pp. 1–26
- Sandhya J, Kalaiselvam S (2020) Biogenic synthesis of magnetic iron oxide nanoparticles using inedible borassus flabellifer seed coat: characterization, antimicrobial, antioxidant activity and in vitro cytotoxicity analysis Mater. Res Express. https://doi.org/10.1088/2053-1591/ab6642
- Alvi, G. B. et al. Biogenic selenium nanoparticles (SeNPs) from citrus fruit have anti-bacterial activities. Rep. 11, 1–11 (2021).
- Sathiyavimal, S. et al. Green chemistry route of biosynthesized copper oxide nanoparticles using Psidium guajava leaf extract and their antibacterial activity and effective removal of industrial dyes. Environ. Chem. Eng. 9, 105033 (2021).
- Jamzad, M. &Bidkorpeh, M. K. Green synthesis of iron oxide nanoparticles by the aqueous extract of Laurus nobilis leaves and evaluation of the antimicrobial activity. J. Nanostruct. Chem. 10, 193–201 (2020).
- Devatha, C., Jagadeesh, K. & Patil, M. Effect of Green synthesized iron nanoparticles by Azardirachta indica in different proportions on antibacterial activity. Nanotechnol. Monit. Manag. 9, 85–94 (2018).
- Ansari, M. A. & Asiri, S. M. M. Green synthesis, antimicrobial, antibiofilm and antitumor activities of superparamagnetic γ-Fe2O3 NPs and their molecular docking study with cell wall mannoproteins and peptidoglycan. J. Biol. Macromol. 171, 44–58 (2021).
- Sadhasivam, S., Vinayagam, V. & Balasubramaniyan, M. Recent advancement in biogenic synthesis of iron nanoparticles. Mol. Struct. 1217, 128372 (2020).
- Ansari, M. A. & Asiri, S. M. M. Green synthesis, antimicrobial, antibiofilm and antitumor activities of superparamagnetic γ-Fe2O3 NPs and their molecular docking study with cell wall mannoproteins and peptidoglycan. J. Biol. Macromol. 171, 44–58 (2021).
- Kirdat, P. et al. Synthesis and characterization of ginger ( officinale) extract mediated iron oxide nanoparticles and its antibacterial activity. Mater. Today Proc. 43, 2826–2831 (2021).
- Thomas, M. D. et al. Too much of a good thing: Adaption to iron(II) intoxication in Escherichia coli. Med. Public Health 9, 53–67 (2021).
- Nwamezie, O. U. I. F. Green synthesis of iron nanoparticles using flower extract of Piliostigmathonningii and their antibacterial activity evaluation. Int. 4, 60–66 (2018).
- Murgueitio, E. et al. Green synthesis of iron nanoparticles: Application on the removal of petroleum oil from contaminated water and soils. Nanotechnol. 2018, 1–8 (2018).
- Vasantharaj, S., Sathiyavimal, S., Senthilkumar, P., LewisOscar, F. & Pugazhendhi, A. Biosynthesis of iron oxide nanoparticles using leaf extract of Ruellia tuberosa: Antimicrobial properties and their applications in photocatalytic degradation. Photochem. Photobiol. B Biol. 192, 74–82 (2019).
- Vasantharaj, S., Sathiyavimal, S., Senthilkumar, P., LewisOscar, F. & Pugazhendhi, A. Biosynthesis of iron oxide nanoparticles using leaf extract of Ruellia tuberosa: Antimicrobial properties and their applications in photocatalytic degradation. Photochem. Photobiol. B Biol. 192, 74–82 (2019).
- Jamzad, M. &Bidkorpeh, M. K. Green synthesis of iron oxide nanoparticles by the aqueous extract of Laurus nobilis leaves and evaluation of the antimicrobial activity. J. Nanostruct. Chem. 10, 193–201 (2020).
- nsari, M. A. & Asiri, S. M. M. Green synthesis, antimicrobial, antibiofilm and antitumor activities of superparamagnetic γ-Fe2O3 NPs and their molecular docking study with cell wall mannoproteins and peptidoglycan. J. Biol. Macromol. 171, 44–58 (2021).
- Kirdat, P. et al. Synthesis and characterization of ginger ( officinale) extract mediated iron oxide nanoparticles and its antibacterial activity. Mater. Today Proc. 43, 2826–2831 (2021).
- Salgado, K. Márquez, O. Rubilar, D. Contreras, and G. Vidal, “The effect of phenolic compounds on the green synthesis of iron nanoparticles (FexOy-NPs) with photocatalytic activity,” Applied Nanoscience, vol. 9, pp. 371–385, 2019.
- Šutka, M. Vanags, A. Spule et al., “Identifying iron-bearings nanoparticles precursor for thermal transformation into the highly active hematite photo-fenton catalyst,” Catalyst, vol. 10, p. 778, 2020.
- Yardily and N. Sunitha, “Green synthesis of iron nanoparticles using hibiscus leaf extract, characterization, antimicrobial activity,” International Journal of Scientific Research and Revie, vol. 8, no. 7, 2019.
- A. Demirezen, S. Yilmaz, and D. D. Yilmaz, “Green synthesis and characterization of iron nanoparticles using aesculus hippocastanum seed extract,” International Journal of Advances in Science Engineering and Technology, vol. 6, 2 pages, 2018.
- Azizi, A. Green synthesis of Fe3O4 nanoparticles and its application in preparation of Fe3O4/cellulose magnetic nanocomposite: A suitable proposal for drug delivery systems. Inorg. Organomet. Polym. Mater. 30, 3552–3561 (2020).
- Bhavyasree, P. G. & Xavier, T. S. Green synthesis of Copper Oxide/Carbon nanocomposites using the leaf extract of Adhatodavasica Nees, their characterization and antimicrobial activity. Heliyon 6, e03323 (2020).
- Nabati Souha, L., Alebrahim, M. T., Habibi Yangjeh, A. &Feizpoor, S. Green synthesis of iron oxide nanoparticles (Fe3O4) by extract of aerial organs of Russian knapweed (Acroptilon repens L.). Mol. Res. (Iranian J. Biol. (2021).
- Alabdallah, N. M. et al. Green synthesized metal oxide nanoparticles mediate growth regulation and physiology of crop plants under drought stress. Plants 10, 1730 (2021).
- Kulak, M. Recurrent drought stress effects on essential oil profile of Lamiaceae plants: An approach regarding stress memory. Crops Prod. 154, 112695 (2020).
- Bardhan, K. Pal, S. Roy et al., “Nanoparticle size-dependent antibacterial activities in natural minerals,” Journal of Nanoscience and Nanotechnology, vol. 11, pp. 7112–7122, 2019.
- Noor, R. et al. Comparative analysis of iron oxide nanoparticles synthesized from ginger (Zingiber officinale) and cumin seeds (Cuminum cyminum) to induce resistance in wheat against drought stress. Chemosphere 292, 133201 (2022).
- Abdelmalek, Z., D’Orazio, A. &Karimipour, A. The Effect of Nanoparticle Shape and Microchannel Geometry on Fluid Flow and Heat Transfer in a Porous Microchannel. Symmetry 12 (2020).
- Inagaki, M., Furuhashi, H., Ozeki, T., et al. “Heat treatment of mesocarbon microbeads under high pressure.” Journal of Materials Science, vol. 6, p. 1520, 1971.
- Inagaki, M., Furuhashi, H., Ozeki, T., & Naka, S. “Integrated intensity changes for crystalline powders by grinding and compression changes in effective temperature factor.” Journal of Materials Science, vol. 8, p. 312, 1973.
- Sirdeshmukh, D. B., Sirdeshmukh, L., & Subhadra, K. G. “Micro-and Macro-properties of Solids.” Springer Series in Materials Science, 2006.
- Gopi Krishna, N., Sirdeshmukh, D. B., Rama Rao, B., Beandry, B. J., &Gschneidner, K. A. “Mean square amplitudes of vibration and associated Debye temperatures of dysprosium, gadolinium, lutetium, and yttrium.” Indian Journal of Pure & Applied Physics, vol. 24, pp. 324–326, 1986.
- Chipman, D.R., and Paskin, A., J.Appl. Phys. 30, (1959) 1938.
- Klug, H.P., and Alexander, L.E., (1974). X-ray Diffraction Procedures (John Wiley and Sons, U.S.A.).
- Cromer, D.T., and Waber, J.T., Acta Cryst. 18, (1965) 104.
- International Tables for X-ray Crystallography (1968) Vol. III (Kynoch Press, Birmingham).
- Cromer, D.T., and Liberman, D., J. Chem. Phys. 53, (1970) 1891.
- Benson, G.C., and Gill, E.K., (1966) Table of Integral Functions Related to Debye-Waller factor, National Research Council of Canada, Ottawa.
- Purushotham, E. “Effect of particle size and lattice strain on the Debye-Waller factors of Fe3C nanoparticles.” Bulletin of Material Science, vol. 37, no. 4, pp. 773–778, 2014.
- Purushotham, E. “Effect of particle size and lattice strain on the Debye-Waller factors of Fe3C nanoparticles.” Bulletin of Material Science, vol. 37, no. 4, pp. 773–778, 2014.
- Purushotham, E., & Gopi Krishna, N. “X-Ray determination of crystallite size and effect.” Chemistry and Materials Research, vol. 7, no. 2, pp. 1–6, 2015.
- Purushotham, E. “Effect of particle size and lattice strain on the Debye-Waller factors of silicon carbide nanoparticles.” Journal of Nanoscience and Nanotechnology, vol. 16, no. 5, pp. 2658–2662, 2016.
- Purushotham, E. “Preparation and characterization of CuInSe2 nanoparticles.” Rasayan Journal of Chemistry, vol. 12, no. 4, pp. 1676–1680, 2019.
- Purushotham, E., & Gopi Krishna, N. “Mean square amplitudes of vibration and associated Debye temperatures of rhenium, osmium, and thallium.” Physica B: Condensed Matter, vol. 405, no. 16, pp. 3308–3311, 2010.
- Purushotham, E., & Gopi Krishna, N. “Effect of lattice strain on the Debye-Waller factors of Mg, Zn, and Cd.” Indian Journal of Physics, vol. 84, no. 7, pp. 887–893, 2010.
- Kaelble, E.F., Handbook of X-rays (New York Mc Graw ill) (1967).
- Purushotham, E. “Mechanical and thermal properties of strained metal nanoparticles prepared by ball milling method.” Rasayan Journal of Chemistry, vol. 13, no. 4, pp. 1676–1680, 2020.
- Vetelino, J. F., Gaur, S. P., & Mitra, S. S. “Lattice Dynamics of Cesium Chloride.” Physical Review B, vol. 5, p. 2360, 1972.
- Glyde, H. R. “Relation of vacancy formation and migration energies to the Debye temperature in solids.” Journal of Physics and Chemistry Solids, vol. 28, p. 2061, 1967.
- Purushotham, E. “Chemical Papers.” Springer Nature, vol. 76, pp. 7327–7331, 2022.
- Purushotham, E. “Evaluation of Debye temperatures of α-phase copper–zinc alloys by using X-Ray diffraction method.” Materials Today Proceedings, vol. 46, no. 12, pp. 5922–5926, 2021.
- Purushotham, E. “Root mean square amplitudes of vibration and associated Debye temperatures of beryllium, scandium, and ruthenium.” Materials Today: Proceedings, vol. 47, no. 15, pp. 5034–5037, 2021.
- Purushotham, E., &Veerati Radhika. “Materials Today: Proceedings.” Elsevier, vol. 47, no. 15, pp. 4993–4995, 2021.
- Purushotham, E. “X-Ray Determination of Debye Temperature and Microhardness of Some Hexagonal Close Packed Elements Re, Os and Tl.” IOP Conference Series: Materials Science and Engineering, vol. 1119, p. 012001, 2021.
- Purushotham, E. “Characterization of size-dependent thermal properties in strained nanocrystalline powder using Williamson-Hall.” IOP Conference Series: Materials Science and Engineering, vol. 981, p. 022086, 2020.

Journal of Polymer and Composites
| Volume | 11 |
| Special Issue | 12 |
| Received | October 30, 2023 |
| Accepted | February 2, 2024 |
| Published | April 1, 2024 |